Status and prospect of in-situ remediation technologies applied in hexavalent chromium contaminated sites
-
摘要:
六价铬Cr(Ⅵ)是一种来源广泛的重金属污染物,因其形态、价态多样,地球化学反应过程极其复杂,同时国内Cr(Ⅵ)污染场地众多,对其修复具有很大的挑战性。原位修复因不开挖土方、对环境干扰小等诸多优势逐渐成为污染场地修复策略的主流。综述了国内外Cr(Ⅵ)不同类型原位修复技术研究进展,在结合大量原位修复工程案例的基础上重点分析了原位生物、原位化学修复技术与不同注入方式的应用效果。阐明了不同类型原位修复技术的适用地层条件、适用浓度范围、影响半径、修复工期、修复介质、药剂类型及用量与施加方式等关键参数。针对复杂的Cr(Ⅵ)污染场地,建立精准的污染场地概念模型,选取高效适宜的修复药剂和注入方式或者修复工艺组合,是确保修复效果的关键。通过分析现有Cr(Ⅵ)不同原位修复技术在实际应用中的优缺点,探索其在不同场景的适用性,同时对技术发展方向进行了展望。
Abstract:Hexavalent chromium Cr(Ⅵ) is a typical heavy metal pollutant with a wide range of sources. Due to its diverse forms and valence states, the geo-chemical reaction process is extremely complicated. There are lots of Cr(Ⅵ) contaminated plots in China and the remediation of Cr(Ⅵ) contaminated sites is very challenging. In-situ remediation has gradually become the mainstream of remediation strategies for contaminated sites due to many advantages such as no excavation and less environmental interference. The latest research progress of different in-situ remediation technologies for Cr(Ⅵ) was reviewed. Based on a large number of in-situ remediation engineering cases at home and abroad, the application effects of in-situ biological, in-situ chemical and other remediation technologies and different injection methods were analyzed. The key parameters of different types of in-situ remediation technologies including applicable geological conditions, applicable concentration range, influence radius, remediation duration, remediation medium, agent type and dosage, and injection method were clarified. It played a key role to establish a precise contamination field concept model, and choose efficient chemicals and the best injection method or remediation process combinations for complex contaminated sites. The advantages and disadvantages of different in-situ remediation technologies of Cr(Ⅵ) were compared in examples, and their different applicable scenarios were explored. At the same time, the future development direction of technologies was prospected.
-
Key words:
- hexavalent chromium /
- in-situ remediation /
- injection /
- reduction agents and materials /
- contaminated site /
- soil /
- groundwater
-
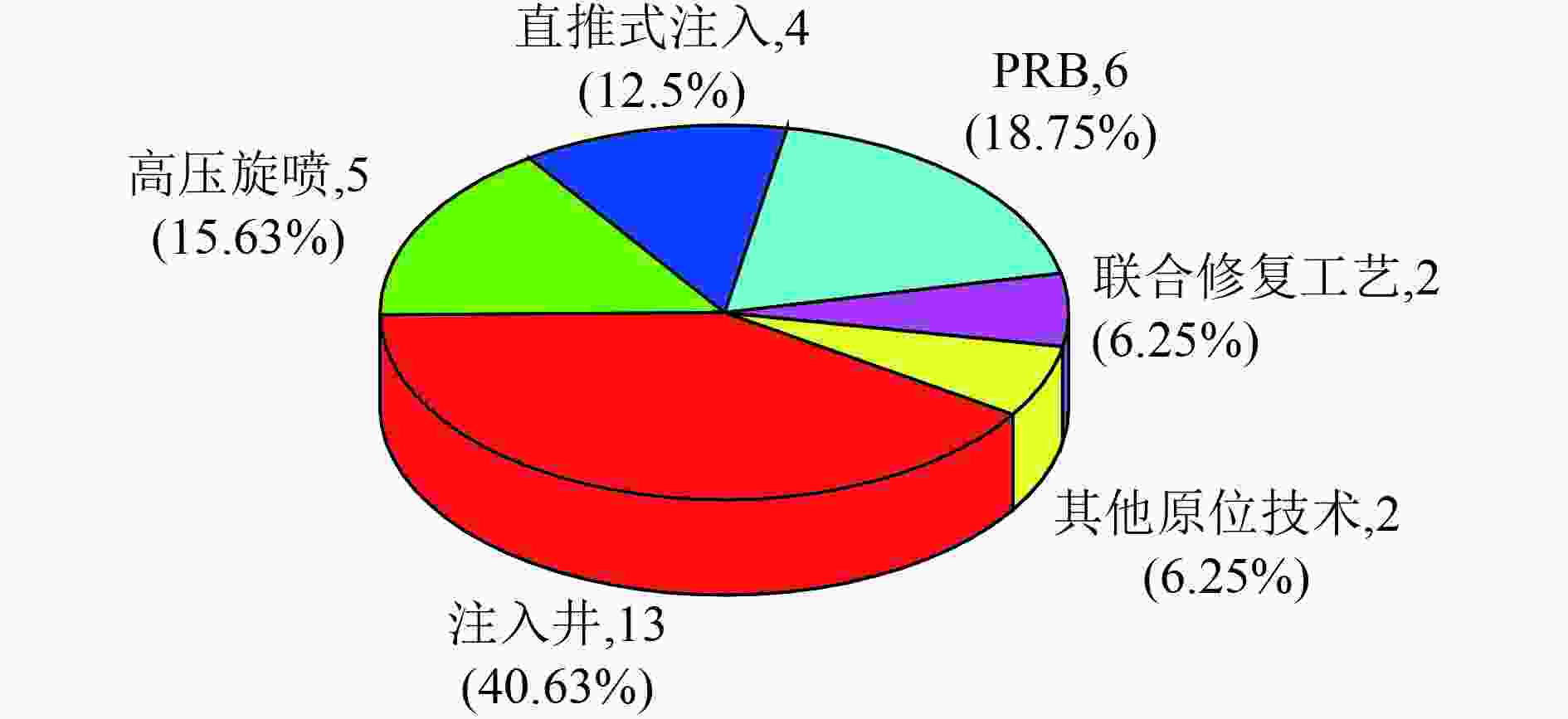
表 1 国内Cr(Ⅵ)原位修复典型案例汇总
Table 1. Summaries of Cr(Ⅵ) in-situ remediation cases at home
类型 项目所在地 规模 岩性 修复介质 原始浓度 修复方式及关键参数 药剂与材料 修复时间 修复效果与存在的问题 原位
化学重庆[46] 中试,修复深度
20 m填土层、泥岩层、砂岩层 土壤 0.30~1.61 mg/kg 场地中部注入井,下游外侧高压旋喷联合工艺 还原药剂、稳定
药剂3 d Cr(Ⅵ)浓度<0.3 mg/kg 山东[47] 中试 粉土和粉黏土 土壤 最高浓度4 570 mg/kg 原位注入,化学还原 多硫化钙 60 d Cr(Ⅵ)浓度<9 mg/kg,去除效果明显,达
标难天津[44] 586 m2,污染深度2~6 m,土方量为4 282 m3;地下水修复范围为1 765 m2 粉黏土层,渗透性较差 土壤 17.1~26.8 mg/kg 高压旋喷技术,有效半径可达1.4 m 硫酸亚铁 Cr(Ⅵ)浓度<2.4 mg/kg 重庆[48] 修复面积为12 246 m2,修复方量为16 150.5 m3,回填土20 m 以下 土壤 最高浓度 1.61 mg/kg 加压注入井+高压旋喷,影响半径分别为2.5和1 m 还原剂与稳定剂 修复后Cr(Ⅵ)浓度<0.25 mg/kg 南方某
地[49]长15 m、宽 12 m、深4 m,720 m3 土壤 污染深度为3~12 m,试验区浓度为273~1 540 mg/kg 原位淋洗与水平井 清水、硫酸亚铁 10批次 Cr(Ⅵ)去除率为93.6%~98.1%,浓度为17.4~29 mg/kg 湖南[50] 20 m2(4 m×5 m),8个注射点,修复深度为地下1~6 m 土壤 1 060~1 540 mg/kg 高压旋喷注射:3%药剂投加比,注射压力10 MPa,影响半径0.5 m,提升速率10 cm/min 硫铁矿物复配稳定化剂 7 d Cr(Ⅵ)浓度<0.5 mg/kg,Cr(Ⅵ)浸出浓度<0.004 mg/L 山东[51] 21 900 m2,最深污染深度48 m 填土层、强风化、中风化、弱风化闪长岩层 地下水 0.12~25 900 mg/L 注入井原位化学还原 硫酸亚铁 400 d 150 d后Cr(Ⅵ)浓度降至0.1 mg/L,第250~400天一直稳定在0.1 mg/L以下,中间出现反弹 国内某
地[42]中试规模 地下水 40~160 mg/L 注入井,影响半径2~4 m 柠檬酸+多硫化钙 20 d 下游Cr(Ⅵ)去除率接近100%,两侧去除率为12.92%~82.22%,下游修复效果明显优于两侧修复效果 北京[43] 6 m×6 m 地下水 1.93 mg/L 直推式注入:GP钻机加压泵注入300 L 5 mg/L的纳米零价铁,影响半径3 m 零价铁 26 d Cr(Ⅵ)浓度为0.001 mg/L,去除率大于99% 西北地
区[17]注射区域面积20 m×10 m,修复深度为17 m 黄土、砾砂、粉黏、粉砂、圆砾 土壤和地下水 地下水:12.15 mg/L;
土壤:100.51 mg/kg锚固旋喷钻机原位注入,压力30 MPa,钻杆提升速度小于0.14 m/min,药剂注入量250 L/m,药剂影响扩散半径0.75 m,钻孔间距1.3 m 石硫合剂 地下水Cr(Ⅵ)浓度<0.01 mg/L;修复后土壤Cr(Ⅵ)浓度平均值为2.7 mg/kg 化学+微生物 山东[47] 中试 粉土和粉黏土 土壤 土壤最高浓度2 730 mg/kg 原位注入,生物化学还原 还原剂、生物营养剂 60 d 土壤Cr(Ⅵ)浓度<70 mg/kg,去除效果明显,达标难 江苏[52] 600 m2,修复深度8 m 粉黏土、黏粉土、砂土 地下水 土壤:200~500 mg/L;地下水:390~456 mg/L 注入井,井间距5 m 还原剂(多硫化钙)、渗透剂、营养剂(乳酸乙酯)、微生物调节剂 37 d 下游地下水Cr(Ⅵ)浓度明显下降,观测井最高去除率为94%~95%。两侧及上游区域修复效果差 山东[53] 杂填土、粉黏土、淤泥质粉黏土 土壤和地下水 土壤60~890 mg/L;
地下水:2.0~12 mg/L高压旋喷,影响半径0.8~1.0 m 硫酸亚铁+自制生物药剂 7 d Cr(Ⅵ)还原率为97.33%~98.96%,土壤和地下水Cr(Ⅵ)浓度为未检出。局部土壤承载力下降,地面下沉 原位微
生物湖南[54] 500 m2,修复深度
24 m砂/卵石、基岩裂隙 地下水 1 000~2 000 mg/L 原位生物刺激+地下水循环井(抽注结合) 乙醇 52 d 修复后Cr(Ⅵ)浓度<0.1 mg/L PRB
(物化)湖南[55] 松散岩类孔隙和基岩裂隙 地下水 0.08~323.24 mg/L PRB:长50 m ,宽2 m,深20 m 填料:零价铁、陶瓷、活性炭 6个月 运行24周,Cr(Ⅵ)浓度均低于修复目标限值0.1 mg/L,25周开始,Cr(Ⅵ)浓度超过目标限值,更换吸附填料 河南[45] 中试 0~8 m和12~15 m岩性为粉黏土至黏土;8~12 m为中细砂 地下水 4~32.4 mg/L PRB:长15 m,宽2.8 m,深12 m 填料:铸铁、活性炭、河沙 10个月 墙体内部Cr(Ⅵ)无检出,去除效果明显;下游浓度由西向东呈高、低、高、低变化,浓度为4~20 mg/L 湖南[56] 240 m2 回填层、黏土层、砂土层、砾石层 地下水 27.29~242.65 mg/L PRB:长27 m,宽3.4 m,深15 m 填料:零价铁、砾石、细沙 6个月(该时间为示范周期,非修复时间) 填料区Cr(Ⅵ)去除率为69%~100%,下游监测井D1、D2和D3的Cr(Ⅵ)浓度分别为16.54~84.75、26.94~96.71和3.65~
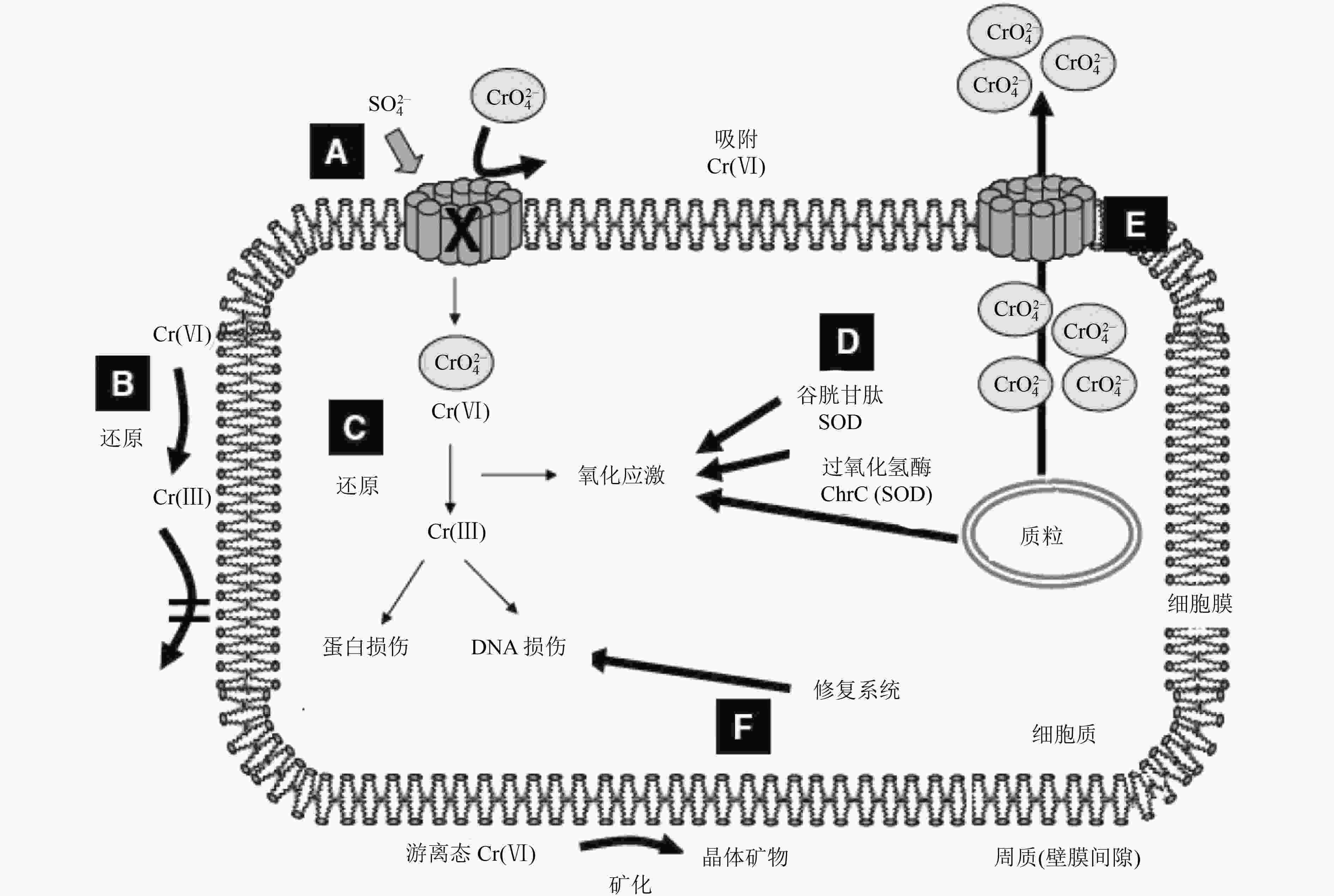
130.27 mg/L,有消减未达标表 2 国外Cr(Ⅵ)原位修复典型案例汇总
Table 2. Summaries of Cr(Ⅵ) in-situ remediation cases abroad
类型 项目所在地 规模 岩性 修复介质 原始浓度 修复方式及关键参数 药剂与材料 修复时间 修复效果与存在的问题 原位化学 美国内华达州[57] 污染深度8~10 m 粉土、粉砂、砂粉 地下水 0.012~0.029 mg/L 原位化学还原:组井注入,分层监测。影响半径5~6 m 多硫化钙 2月 Cr(Ⅵ)去除率为67%~99% 奥地利[58] 约1000 m2 砾石、细砂、粉黏土 地下水 0.2~2 mg/L 上游建注入井 连二亚硫酸钠 240 d Cr(Ⅵ)浓度从2 mg/L降低至0.1 mg/L以下,但是停止注入后出现
反弹美国南卡罗来纳
州[22]修复深度5 m 地下水 5~52 mg/L Geoprobe直推式注入;影响半径1.75~2.5 m 连二亚硫酸钠+硫酸亚铁 40 d 影响半径范围内地下水Cr(Ⅵ)未检出 美国南卡罗来纳
州[21]150 m2;修复深度3.0~4.5 m 地下水 4.8 ~7.4 mg/L Geoprobe直推式注入,注入压力0.20 MPa 硫酸亚铁 + 连二亚硫酸钠 120 d 120 d后检测,地下水中Cr(Ⅵ)浓度<0.01 mg/L,修复797 d后土壤中仍有检出,平均浓度为0.382 mg/kg 意大利东北部[59] 地下水 4 500 μg/L 自然衰减 6 a 自然衰减6 a后无检出。但是再过由于锰的氧化作用5 a后出现反弹。地下水Cr(Ⅵ)浓度达到1 560 μg/L 英国肖菲尔德[19] 中试25 m2,113 m3,修复深度1.5~6 m 土壤和地下水 土壤:148 mg/kg;地下水:7.42 mg/L 地下水循环井,抽提处理后回注 多硫化钙 23 d 土壤中Cr(Ⅵ)降低了86%,地下水去除率接近100% 英国肖菲尔德[19] 中试24 m2,230 m3,深度0.4~9.6 m 土壤和地下水 土壤:354 mg/kg;地下水:0.835 mg/L 直推式注入,影响半径1 m 多硫化钙 21 d Cr(Ⅵ)去除率接近100% 英国肖菲尔德[19] 中试25 m2,250 m3,深度0~10 m 土壤和地下水 土壤:342 mg/kg;
地下水:143 μg /L高压旋喷 多硫化钙 18 d 土壤修复效果好,Cr(Ⅵ)去除率100% 原位生物 瑞士图恩[60] 30 m2,深度7 m,污染羽长度250 m 河流冲击砂层 地下水 2.5~4 mg/L 原位生物刺激:注入井注入,分批次间歇式注入 糖蜜 1 a 注入2个月,Cr(Ⅵ)浓度降低至为0.005~0.5 mg/L,停止注药后稳定 美国内华达州[52] 最深18 m 粉土、粉砂、砂粉 地下水 11 mg/L 原位生物还原/刺激:组井注入,分层监测,注入压力0.12 MPa。影响半径5~6 m 碳源:糖粒,糖蜜,糖业废水;氮磷助剂:磷酸二氢钠,尿素,碳酸氢钠 2月,3批次注药 Cr(Ⅵ)浓度从11 mg/L降至0.01 mg/L 美国华盛顿[29] 30 m×30 m,修复深度15.84 m 0~12.2 m粗砂,以下为粉土、粉黏土 地下水 10 mg/L 循环井原位生物刺激:注入井注入,下游设置抽提井(不连续抽提)。影响半径5 m 甘油、聚乳酸 1 a 地下水Cr(Ⅵ)未检出,注药3 a后依然稳定 比利时法兰德斯[61] 中试规模8~12 m 地下水 最高浓度 80 000 µg/L 注入井,原位生物刺激 糖蜜和乳酸盐 410 d 修复后Cr(Ⅵ)浓度低于50 µg/L PRB 瑞士图恩[62] 高渗透地层,碳酸盐砾石含水层 地下水 4 mg/L PRB,单桩直径1.3 m,修复深度
13 m铁屑、砾石 2 a 运行2 a内大部分Cr(Ⅵ)绕流PRB,下游无修复效果 瑞士维利绍[63] 中试规模,12~
23 m地下水 0~2.5 mg/L PRB,填充桩直径1.3 m 零价铁 4 a 双排桩可有效治理下游地下水Cr(Ⅵ),单排桩修复效果差 美国北卡罗来纳
州[64-65]地下水 5 mg/L PRB,反应墙长45.7 m、
深7.3 m、厚0.6 m零价纳米铁 2 a 修复后Cr(Ⅵ)浓度降至未检出 -
[1] TANG X, HUANG Y, LI Y, et al. Study on detoxification and removal mechanisms of hexavalent chromium by microorganisms[J]. Ecotoxicology and Environmental Safety,2021,208:111699. doi: 10.1016/j.ecoenv.2020.111699 [2] US EPA. Superfund remedy report 16th edition: EPA-542-R-20-001[R]. Washington DC: Office of Land and Emergency Management, 2020. [3] 生态环境部土壤生态环境司, 生态环境部土壤与农业农村生态环境监管技术中心, 生态环境部南京环境科学研究所. 地下水污染风险管控与修复技术手册[M]. 北京: 中国环境出版集团, 2021. [4] 倪碧珩, 施维林, 陈洁, 等.某电镀厂地块重金属污染特征与健康风险空间分布评价[J]. 环境工程技术学报,2022,12(3):878-885. doi: 10.12153/j.issn.1674-991X.20210142NI B H, SHI W L, CHEN J, et al. Pollution characteristics and spatial distribution evaluation of the health risk of heavy metals in an electroplating plant site[J]. Journal of Environmental Engineering Technology,2022,12(3):878-885. doi: 10.12153/j.issn.1674-991X.20210142 [5] SUN H, BROCATO J, COSTA M. Oral chromium exposure and toxicity[J]. Current Environmental Health Reports,2015,2(3):295-303. doi: 10.1007/s40572-015-0054-z [6] TASSI E, GRIFONI M, BARDELLI F, et al. Evidence for the natural origins of anomalously high chromium levels in soils of the Cecina Valley (Italy)[J]. Environmental Science Processes & Impacts,2018,20(6):965-976. [7] PATERNOSTER M, RIZZO G, SINISI R, et al. Natural hexavalent chromium in the pollino massif groundwater (southern Apennines, Italy): occurrence, geochemistry and preliminary remediation tests by means of innovative adsorbent nanomaterials[J]. Bulletin of Environmental Contamination and Toxicology,2021,106(3):421-427. doi: 10.1007/s00128-020-02898-7 [8] 王兴润, 李磊, 颜湘华, 等.铬污染场地修复技术进展[J]. 环境工程,2020,38(6):1-8.WANG X R, LI L, YAN X H, et al. Progress in remediation of chromium-contaminated sites[J]. Environmental Engineering,2020,38(6):1-8. [9] 陈志良, 周建民, 蒋晓璐, 等.典型电镀污染场地重金属污染特征与环境风险评价[J]. 环境工程技术学报,2014,4(1):80-85.CHEN Z L, ZHOU J M, JIANG X L, et al. Pollution characteristics and environmental risk assessment of heavy metals in typical electroplating contaminated site[J]. Journal of Environmental Engineering Technology,2014,4(1):80-85. [10] WANG X R, LI L, YAN X H, et al. Processes of chromium (Ⅵ) migration and transformation in chromate production site: a case study from the middle of China[J]. Chemosphere,2020,257:127282. doi: 10.1016/j.chemosphere.2020.127282 [11] 周建军, 马宏瑞, 朱超, 等.制革污泥铬的形态与危险废物识别方法[J]. 化工进展,2017,36(4):1476-1481.ZHOU J J, MA H R, ZHU C, et al. Cr speciation and hazardous waste identification in tannery sludge[J]. Chemical Industry and Engineering Progress,2017,36(4):1476-1481. [12] XIA S P, SONG Z L, JEYAKUMAR P, et al. Characteristics and applications of biochar for remediating Cr(Ⅵ)-contaminated soils and wastewater[J]. Environmental Geochemistry and Health,2020,42(6):1543-1567. doi: 10.1007/s10653-019-00445-w [13] 孟凡生, 王业耀, 李莉.PRB去除模拟地下水中六价铬的反应特性[J]. 环境工程技术学报,2013,3(2):92-97.MENG F S, WANG Y Y, LI L. Reactivity characteristics of hexavalent chromium removed by PRB in simulated ground water[J]. Journal of Environmental Engineering Technology,2013,3(2):92-97. [14] KANTAR C, CETIN Z, DEMIRAY H. In situ stabilization of chromium(Ⅵ) in polluted soils using organic ligands: the role of galacturonic, glucuronic and alginic acids[J]. Journal of Hazardous Materials,2008,159(2/3):287-293. [15] TAKENO N. Intercomparison of thermodynamic databases[R]. Tsukuba: National Institute of Advanced Industrial Science and Technology, 2005: 153-155. [16] FARMER J G, THOMAS R P, GRAHAM M C, et al. Chromium speciation and fractionation in ground and surface waters in the vicinity of chromite ore processing residue disposal sites[J]. Journal of Environmental Monitoring:JEM,2002,4(2):235-243. doi: 10.1039/b108681m [17] 中关村中环地下水污染防控与修复产业联盟. 污染地下水原位注入修复技术指南: T/GIA 002—2019[S]. 北京: 中国标准出版社, 2019. [18] THORNTON E C, AMONETTE J E. Hydrogen sulfide gas treatment of Cr(Ⅵ)-contaminated sediment samples from a plating-waste disposal SiteImplications for in situ remediation[J]. Environmental Science & Technology,1999,33(22):4096-4101. [19] CLAIR E. In situ deliverability trials using calcium polysulphide to treat chromium contamination at Shawfield[R]. Glasgow: TDP Bulleti, 2013. [20] JAMES B R. Peer reviewed: the challenge of remediating chromium-contaminated soil[J]. Environmental Science & Technology,1996,30(6):248A-251A. [21] LUDWIG R D, SU C M, LEE T R, et al. In situ chemical reduction of Cr(Ⅵ) in groundwater using a combination of ferrous sulfate and sodium dithionite: a field investigation[J]. Environmental Science & Technology,2007,41(15):5299-5305. [22] SU C M, LUDWIG R D. Treatment of hexavalent chromium in chromite ore processing solid waste using a mixed reductant solution of ferrous sulfate and sodium dithionite[J]. Environmental Science & Technology,2005,39(16):6208-6216. [23] PAN C, LIU H, CATALANO J G, et al. Rates of Cr(Ⅵ) generation from CrxFe1-x(OH)3 solids upon reaction with manganese oxide[J]. Environmental Science & Technology,2017,51(21):12416-12423. [24] PAN C, TROYER L D, CATALANO J G, et al. Dynamics of chromium(Ⅵ) removal from drinking water by iron electrocoagulation[J]. Environmental Science & Technology,2016,50(24):13502-13510. [25] LIN Y, CAI W, TIAN X, et al. Polyacrylonitrile/FeCl2 composite nanofibers, fabricated by electrospinning, exhibited excellent performance for Cr-removal from Cr2O72−-containing solutions in one step[J]. Journal of Materials Chemistry,2011,21:991-997. doi: 10.1039/C0JM02334E [26] 刘美丽, 牛其建, 俞洋洋, 等.碳基材料负载纳米零价铁去除六价铬的研究进展[J]. 环境科学研究,2022,35(3):768-779.LIU M L, NIU Q J, YU Y Y, et al. Progress in removal of hexavalent chromium by carbon-based materials loaded with nano zero-valent iron[J]. Research of Environmental Sciences,2022,35(3):768-779. [27] RIVERO-HUGUET M, MARSHALL W D. Reduction of hexavalent chromium mediated by micron- and nano-scale zero-valent metallic particles[J]. Journal of Environmental Monitoring:JEM,2009,11(5):1072-1079. doi: 10.1039/b819279k [28] SEAMAN J C, BERTSCH P M, SCHWALLIE L. In situ Cr(Ⅵ) reduction within coarse-textured, oxide-coated soil and aquifer systems using Fe(Ⅱ) solutions[J]. Environmental Science & Technology,1999,33(6):938-944. [29] FAYBISHENKO B, HAZEN T C, LONG P E, et al. In situ long-term reductive bioimmobilization of Cr(Ⅵ) in groundwater using hydrogen release compound[J]. Environmental Science & Technology,2008,42(22):8478-8485. [30] RAMÍREZ-DÍAZ M I, DÍAZ-PÉREZ C, VARGAS E, et al. Mechanisms of bacterial resistance to chromium compounds[J]. BioMetals,2008,21(3):321-332. doi: 10.1007/s10534-007-9121-8 [31] SUKLA L B, PRADHAN N, PANDA S, et al. Environmental microbial biotechnology[M]. Cham: Springer International Publishing, 2015. [32] JOBBY R, JHA P, YADAV A K, et al. Biosorption and biotransformation of hexavalent chromium (Cr(Ⅵ)): a comprehensive review[J]. Chemosphere,2018,207:255-266. doi: 10.1016/j.chemosphere.2018.05.050 [33] AHMAD W A, VENIL C K, NKHALAMBAYAUSI CHIRWA E M, et al. Bacterial reduction of Cr(Ⅵ): operational challenges and feasibility[J]. Current Pollution Reports,2021,7(2):115-127. doi: 10.1007/s40726-021-00174-8 [34] TANG R B, SHEN L H, YANG L, et al. Killing two birds with one stone: biomineralized bacteria tolerate adverse environments and absorb hexavalent chromium[J]. ACS Omega,2022,7(18):15385-15395. doi: 10.1021/acsomega.1c06877 [35] XIA S P, SONG Z L, JEYAKUMAR P, et al. A critical review on bioremediation technologies for Cr(Ⅵ)-contaminated soils and wastewater[J]. Critical Reviews in Environmental Science and Technology,2019,49(12):1027-1078. doi: 10.1080/10643389.2018.1564526 [36] SINGH P, ITANKAR N, PATIL Y. Biomanagement of hexavalent chromium: current trends and promising perspectives[J]. Journal of Environmental Management,2021,279:111547. doi: 10.1016/j.jenvman.2020.111547 [37] 闫潇, 王建雷, 张明江, 等.微生物修复返溶铬污染场地的研究进展[J]. 生物工程学报,2021,37(10):3591-3603.YAN X, WANG J L, ZHANG M J, et al. Advances in microbial remediation of the re-dissolved chromium contaminated sites[J]. Chinese Journal of Biotechnology,2021,37(10):3591-3603. [38] 施国静, 吴效俭, 王莹莹.细菌六价铬还原及吸附机制研究进展[J]. 微生物学报,2022,62(11):4287-4304. doi: 10.13343/j.cnki.wsxb.20220144SHI G J, WU X J, WANG Y Y. Mechanism of bacterial reduction and biosorption of hexavalent chromium[J]. Acta Microbiologica Sinica,2022,62(11):4287-4304. doi: 10.13343/j.cnki.wsxb.20220144 [39] 夏险, 李明顺, 武士娟, 等.微生物铬转化和抗性机制与生物修复研究进展[J]. 微生物学通报,2017,44(7):1668-1675.XIA X, LI M S, WU S J, et al. Research progress in microbial chromium-transformation and resistance and bioremediation[J]. Microbiology China,2017,44(7):1668-1675. [40] JACOBS J A. In situ remediation of heavy metals in groundwater[J]. Encyclopedia of Water,2005,1:1-5. [41] 王泓泉.污染地下水可渗透反应墙(PRB)技术研究进展[J]. 环境工程技术学报,2020,10(2):251-259. doi: 10.12153/j.issn.1674-991X.20190129WANG H Q. Study on permeable reactive barrier technology for the remediation of polluted groundwater[J]. Journal of Environmental Engineering Technology,2020,10(2):251-259. doi: 10.12153/j.issn.1674-991X.20190129 [42] 李慧芳, 陈文芳, 陈磊磊, 等.原位化学修复技术在某Cr(Ⅵ)污染场地地下水应用研究[J]. 水资源与水工程学报,2022,33(3):81-88. doi: 10.11705/j.issn.1672-643X.2022.03.11LI H F, CHEN W F, CHEN L L, et al. Application of in situ chemical remediation technology to groundwater in a Cr(Ⅵ) contaminated site[J]. Journal of Water Resources and Water Engineering,2022,33(3):81-88. doi: 10.11705/j.issn.1672-643X.2022.03.11 [43] 王棣, 魏文侠, 王琳玲, 等.纳米铁原位注入技术对六价铬污染地下水的修复[J]. 环境工程学报,2018,12(2):521-526. doi: 10.12030/j.cjee.201706140WANG D, WEI W X, WANG L L, et al. Remediation of chromium (Ⅵ) contaminated groundwater by in situ injection of nanoscale zero valent iron[J]. Chinese Journal of Environmental Engineering,2018,12(2):521-526. doi: 10.12030/j.cjee.201706140 [44] 邱沙, 宋景鹏, 陈志国, 等.原位化学还原技术修复铬污染土壤及其工程应用[J]. 环境科学与技术,2021,44(4):131-139. doi: 10.19672/j.cnki.1003-6504.2021.04.017QIU S, SONG J P, CHEN Z G, et al. Remediation of chromium contaminated soil by in situ chemical reduction technology and its engineering application[J]. Environmental Science & Technology,2021,44(4):131-139. doi: 10.19672/j.cnki.1003-6504.2021.04.017 [45] 李敬杰, 蔡五田, 吕永高, 等.中试尺度下连续式可渗透反应墙修复Cr(Ⅵ)污染地下水效果评估[J]. 环境工程,2022,40(2):162-167.LI J J, CAI W T, LÜ Y G, et al. Effect evaluation of Cr(Ⅵ) contaminated groundwater remediation by permeable reactive wall in pilot scale[J]. Environmental Engineering,2022,40(2):162-167. [46] 曹俊, 申源源, 张建, 等.原位修复某大埋深六价铬污染土壤的中试研究[J]. 环境工程,2019,37:921-926.CAO J, SHEN Y Y, ZHANG J, et al. Pilot-scale study on in-situ remediation of a heavily buried hexavalent chromium contaminated soil[J]. Environmental Engineering,2019,37:921-926. [47] 车玉翠.不同铬修复技术中试应用研究[J]. 检验检疫学刊,2020,30(3):114-116.CHE Y C. Piolt study on different remediation technologies of chromium[J]. Journal of Inspection and Quarantine,2020,30(3):114-116. [48] 刘益风, 李洁, 申源源, 等.重庆某六价铬污染场地土壤修复工程案例[J]. 广州化工,2019,47(12):111-114. doi: 10.3969/j.issn.1001-9677.2019.12.040LIU Y F, LI J, SHEN Y Y, et al. Soil remediation engineering instances of a hexavalent chromium contaminated site in Chongqing[J]. Guangzhou Chemical Industry,2019,47(12):111-114. doi: 10.3969/j.issn.1001-9677.2019.12.040 [49] 王廷涛, 郭贝, 赵志辉.铬污染土壤原位修复技术试验研究[J]. 中国环保产业,2021(1):61-64. doi: 10.3969/j.issn.1006-5377.2021.01.013WANG T T, GUO B, ZHAO Z H. Experimental study of the technology for in-situ remediation of chromium-contaminated soil[J]. China Environmental Protection Industry,2021(1):61-64. doi: 10.3969/j.issn.1006-5377.2021.01.013 [50] 邵乐, 刘晓月, 史学峰, 等.原位还原稳定化—高压旋喷注射技术修复铬污染场地中试研究[J]. 环境科学导刊,2018,37(4):54-57. doi: 10.13623/j.cnki.hkdk.2018.04.013SHAO L, LIU X Y, SHI X F, et al. Remediation of chromic-contaminated site by in situ reduction and stabilization-high pressure jet injection technology at pilot-scale studies[J]. Environmental Science Survey,2018,37(4):54-57. doi: 10.13623/j.cnki.hkdk.2018.04.013 [51] 刘松玉, 刘宜昭, 赵洁丽, 等.裂隙岩体含水层六价铬污染的修复[J]. 岩土工程学报,2020,42(3):413-420.LIU S Y, LIU Y Z, ZHAO J L, et al. Remediation of fractured rock aquifers contaminated by hexavalent chromium[J]. Chinese Journal of Geotechnical Engineering,2020,42(3):413-420. [52] 张建荣, 李娟, 许伟.原位生物稳定固化技术在铬污染场地治理中的应用研究[J]. 环境科学,2013,34(9):3684-3689. doi: 10.13227/j.hjkx.2013.09.053ZHANG J R, LI J, XU W. Research on the application of in-situ biological stabilization solidification technology in chromium contaminated site management[J]. Environmental Science,2013,34(9):3684-3689. doi: 10.13227/j.hjkx.2013.09.053 [53] 余定坤, 尹炳奎.高压旋喷在某铬污染场地原位修复中的应用研究[J]. 广东化工,2020,47(10):106. doi: 10.3969/j.issn.1007-1865.2020.10.049YU D K, YIN B K. Study on application of high pressure jet grounting to in-situ repair of a chromium contaminated site[J]. Guangdong Chemical Industry,2020,47(10):106. doi: 10.3969/j.issn.1007-1865.2020.10.049 [54] SONG X, WANG Q, JIN P, et al. Enhanced biostimulation coupled with a dynamic groundwater recirculation system for Cr(Ⅵ) removal from groundwater: a field-scale study[J]. Science of the Total Environment,2021,772:145495. doi: 10.1016/j.scitotenv.2021.145495 [55] 邓江兰, 朱泽民, 叶明强.某铬污染场地地下水可渗透反应墙技术(PRB)修复中试应用[J]. 湖南有色金属,2021,37(5):57-60. doi: 10.3969/j.issn.1003-5540.2021.05.017DENG J L, ZHU Z M, YE M Q. Pilot application of PRB remediation of groundwater in a chromium contaminated site[J]. Hunan Nonferrous Metals,2021,37(5):57-60. doi: 10.3969/j.issn.1003-5540.2021.05.017 [56] WANG Q, SONG X, WEI C L, et al. in situ remediation of Cr(Ⅵ) contaminated groundwater by ZVI-PRB and the corresponding indigenous microbial community responses: a field-scale study[J]. Science of the Total Environment,2022,805:150260. doi: 10.1016/j.scitotenv.2021.150260 [57] In-situ chromium treatability study results report Nevada environmental response trust site Henderson, Nevada[R]. Nevada: Tetra Tech, inc, 2018. [58] SEDLAZECK K P, VOLLPRECHT D, MÜLLER P, et al. Impact of an in situ Cr(Ⅵ)-contaminated site remediation on the groundwater[J]. Environmental Science and Pollution Research,2020,27(13):14465-14475. doi: 10.1007/s11356-019-07513-9 [59] SLEJKO F F, PETRINI R, LUTMAN A, et al. Chromium isotopes tracking the resurgence of hexavalent chromium contamination in a past-contaminated area in the Friuli Venezia Giulia Region, northern Italy[J]. Isotopes in Environmental and Health Studies,2019,55(1):56-69. doi: 10.1080/10256016.2018.1560278 [60] NAZAROVA T, ALESSI D S, JANSSEN D J, et al. In situ biostimulation of Cr(Ⅵ) reduction in a fast-flowing oxic aquifer[J]. ACS Earth and Space Chemistry,2020,4(11):2018-2030. doi: 10.1021/acsearthspacechem.0c00200 [61] VANBROEKHOVEN K, VERMOORTEL Y, DIELS L, et al. Stimulation of in situ bioprecipitation for the removal of hexavalent chromium from contaminated groundwater[C]//IMWA Symposium 2007: Water in Mining Environments. Italy: Cagliari R Cidu & F. Frau (Eds), 2007. [62] WANNER C, ZINK S, EGGENBERGER U, et al. Unraveling the partial failure of a permeable reactive barrier using a multi-tracer experiment and Cr isotope measurements[J]. Applied Geochemistry,2013,37:125-133. doi: 10.1016/j.apgeochem.2013.07.019 [63] FLURY B, EGGENBERGER U, MÄDER U. First results of operating and monitoring an innovative design of a permeable reactive barrier for the remediation of chromate contaminated groundwater[J]. Applied Geochemistry,2009,24(4):687-696. doi: 10.1016/j.apgeochem.2008.12.020 [64] USCG. In situ permeable reactive barrier for treatment of contaminated groundwater at the US Coast Guard Support Center, Elizabeth City, North Carolina [R]. Elizabeth City, NC: US Coast Guard Support Center, 1998. [65] PULS R W, BLOWES D W, GILLHAM R W. Long-term performance monitoring for a permeable reactive barrier at the U. S. Coast Guard Support Center, Elizabeth City, North Carolina[J]. Journal of Hazardous Materials,1999,68(1/2):109-124. [66] BUTLER E C, CHEN L X, HANSEL C M, et al. Biological versus mineralogical chromium reduction: potential for reoxidation by Manganese oxide[J]. Environmental Science Processes & Impacts,2015,17(11):1930-1940. [67] WEN C Y, SHENG H, REN L M, et al. Study on the removal of hexavalent chromium from contaminated groundwater using emulsified vegetable oil[J]. Process Safety and Environmental Protection,2017,109:599-608. doi: 10.1016/j.psep.2017.05.004 [68] ZHANG P, van NOSTRAND J D, HE Z L, et al. A slow-release substrate stimulates groundwater microbial communities for long-term in situ Cr(Ⅵ) reduction[J]. Environmental Science & Technology,2015,49(21):12922-12931. □ -




 下载:
下载: